Research and development
-
Guangzhou Westpoint Pharmatech. Co., Ltd.
Tel:0769-2223 5501
Address: 4th Floor, 8th Building, Industrial Center, No. 19 Alishan Road, Songshan Lake High-tech Industrial Development Zone, Dongguan City, Guangdong Province
Dongguan Westpoint Pharmatech. Co., Ltd.
Tel:0769-2223 5501
Address: 4th Floor, 8th Building, Industrial Center, No. 19 Alishan Road, Songshan Lake High-tech Industrial Development Zone, Dongguan City, Guangdong Province
Jiangsu Westpoint Pharmaceutical Excipients Co., Ltd.
Tel:0523-80103166
Address: West side of 1st to 4th floor of G56 Standard Factory Building of China Pharmaceutical City Phase IV, Hailing District, Taizhou City, Jiangsu Province
Your location:Home > Company's Profile
Company profile
Due to the big quality gap between Chinese pharmaceutical industry and global giant drug makers, the experts with broad experiences in Dongguan WestPoint Pharmatech. Co., Ltd. set up a contract research organization (CRO) for generic drug R&D. It provides services of generic drug registration and application submission in EU, US and China for domestic pharmaceutical enterprises. It helps domestic enterprises speeding up globalization and sell their drug products in global market.
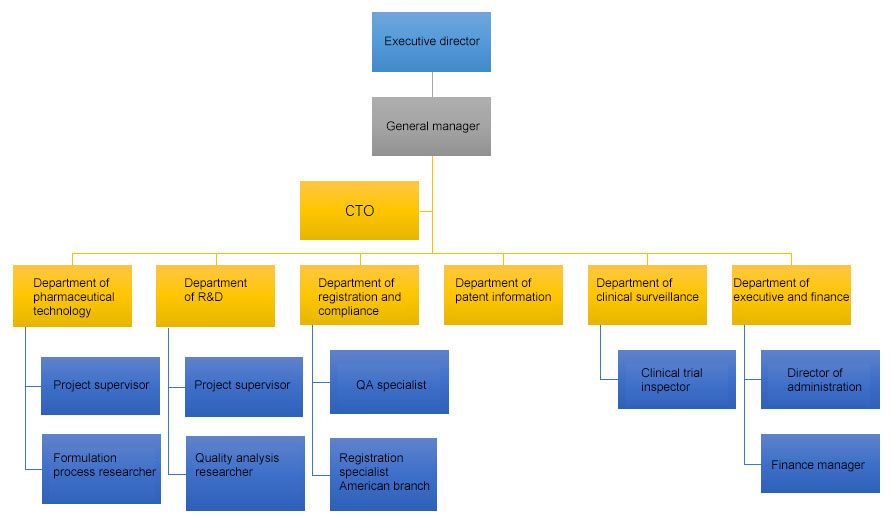
After more than 10 years development, our company has established an global-level generic drug R&D system and project team system to ensure the high efficiency and quality of the project.

Advanced research and development facility: our company has established modern pharmaceutical technology development center in Songshan Lake, Dongguan and Nanjing respectively, and owns advanced testing facilities, equipment and instruments used to manufacture generic drug product from small scale to pilot scale.

Global level technical team and management team: our company has many seasoned scientists and project managers in drug development and registration in EU and US, including doctors specialized in generic drug development in US and R&D personnel with more than 10 years experiences in product development and registration in the United States.
Global level management system and technology system: our company has established a “generic drug consistency evaluation” working system, R&D project management system and project R&D technology working system. All these systems are compliance with the global standard.



 0769-22235501
0769-22235501
